
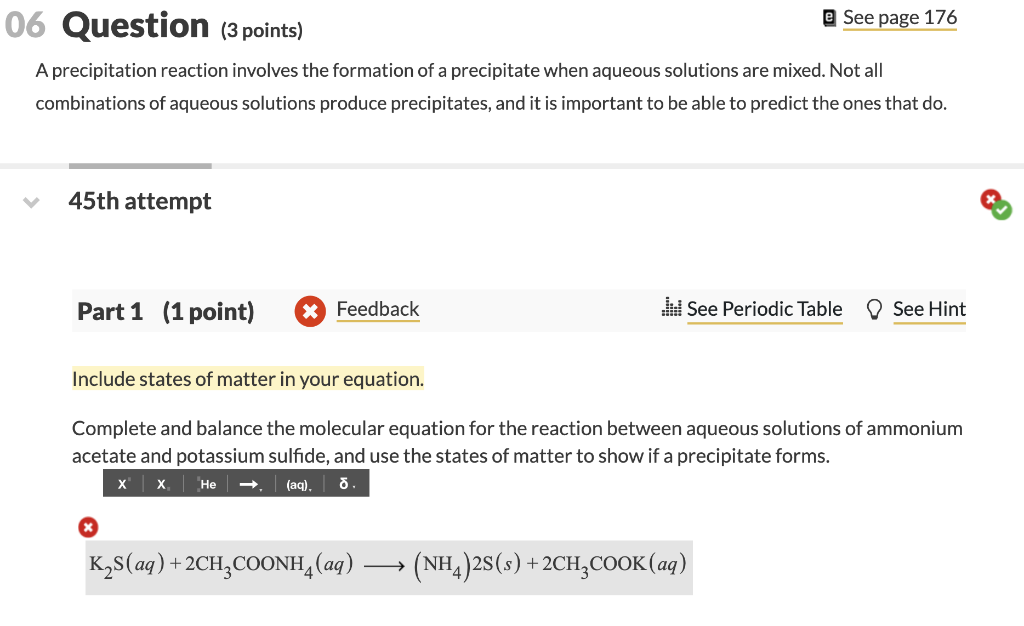
With the increase in research done on Cannabis and its unique compounds, various extraction methods from either hemp fibres or Cannabis flowers are commercially used to produce valuable extracts. It can be concluded that due to the high concentration of cannabinoids remaining in the wax even after processing, and their relative commercial value, recovery of the cannabinoids from the wax could form a potential valorisation application for the underutilised Cannabis wax by-product. The most abundant wax compound class in wax B was the n-alkanes at a concentration of 54.55 mg/g and the dominant species in that fraction was nonacosane (C29) with a concentration of 24.47 mg/g. For wax A the largest remaining wax compound class was the fatty acids, which reported a concentration of 172.2 mg/g, with linoleic acid being the most abundant at a concentration of 68.47 mg/g. The cannabinoid fraction was the most abundant fraction in both waxes, reporting a total fraction of 509.3 mg/g for wax A and 392.6 mg/g for wax B, on a solvent-free basis. The main classes quantified in the Cannabis waxes were cannabinoids, n-alkanes, fatty alcohols, fatty acids, sterols, and various terpenoids. Two industrial wax by-products (wax A and wax B) were used as the feedstock for the characterisation, differing in both strain of Cannabis used and downstream processing conditions. This study applied both gas and liquid chromatography methods to characterise the major compounds present in the waxy by-product from commercial Cannabis processing. Next, learn about the Effects of Acid Rain.Cannabinoid extraction during Cannabis processing produces a wax by-product which is currently underutilised, partially because the composition is poorly understood. The Long-Term Monitoring (LTM) Network measures and monitors surface water chemistry at over 280 sites to provide valuable information on aquatic ecosystem health and how water bodies respond to changes in acid-causing emissions and acid deposition. When acid deposition is washed into lakes and streams, it can cause some to turn acidic. Air concentrations are measured by CASTNET at more than 90 locations. Dry deposition estimates for nitrogen and sulfur pollutants are provided by the Clean Air Status and Trends Network (CASTNET). Unlike wet deposition, dry deposition is difficult and expensive to measure. The NADP/NTN collects acid rain at more than 250 monitoring sites throughout the US, Canada, Alaska, Hawaii and the US Virgin Islands. Policymakers, research scientists, ecologists, and modelers rely on the National Atmospheric Deposition Program’s (NADP) National Trends Network (NTN) for measurements of wet deposition.

Acid rain usually has a pH between 4.2 and 4.4. Normal rain has a pH of about 5.6 it is slightly acidic because carbon dioxide (CO 2) dissolves into it forming weak carbonic acid. The lower a substance's pH (less than 7), the more acidic it is the higher a substance's pH (greater than 7), the more alkaline it is. For example, in desert areas the ratio of dry to wet deposition is higher than an area that receives several inches of rain each year.Īcidity and alkalinity are measured using a pH scale for which 7.0 is neutral. The amount of acidity in the atmosphere that deposits to earth through dry deposition depends on the amount of rainfall an area receives. When the accumulated acids are washed off a surface by the next rain, this acidic water flows over and through the ground, and can harm plants and wildlife, such as insects and fish. The acidic particles and gases may deposit to surfaces (water bodies, vegetation, buildings) quickly or may react during atmospheric transport to form larger particles that can be harmful to human health. Dry DepositionĪcidic particles and gases can also deposit from the atmosphere in the absence of moisture as dry deposition.

The sulfuric and nitric acids formed in the atmosphere fall to the ground mixed with rain, snow, fog, or hail. Wet deposition is what we most commonly think of as acid rain. Winds can blow SO 2 and NO X over long distances and across borders making acid rain a problem for everyone and not just those who live close to these sources. Manufacturing, oil refineries and other industries.

Two thirds of SO 2 and one fourth of NO X in the atmosphere come from electric power generators.
The major sources of SO 2 and NO X in the atmosphere are: While a small portion of the SO 2 and NO X that cause acid rain is from natural sources such as volcanoes, most of it comes from the burning of fossil fuels. These then mix with water and other materials before falling to the ground. The SO 2 and NO X react with water, oxygen and other chemicals to form sulfuric and nitric acids. Acid rain results when sulfur dioxide (SO 2) and nitrogen oxides (NO X) are emitted into the atmosphere and transported by wind and air currents.


 0 kommentar(er)
0 kommentar(er)
